Portsmouth manufacturer Portsmouth Aviation joins exovent task force to take innovative ventilator through to production
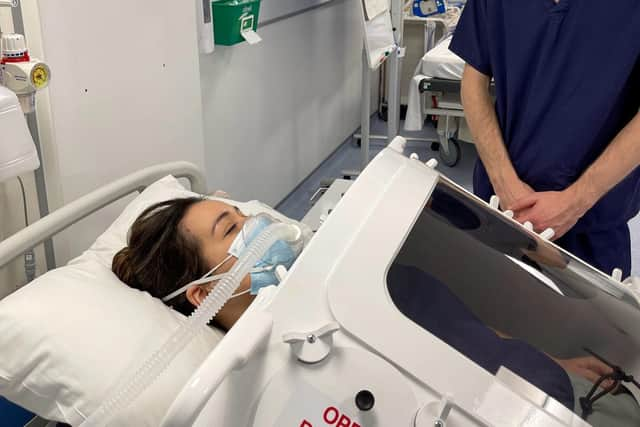

Portsmouth Aviation, in Airport Service Road, is developing the negative pressure ventilator with exovent, a team formed in March 2020 in response to the pandemic and is made up of anaesthetists, critical care consultants, nurses, medical clinicians, engineers, academics, scientists and manufacturers.
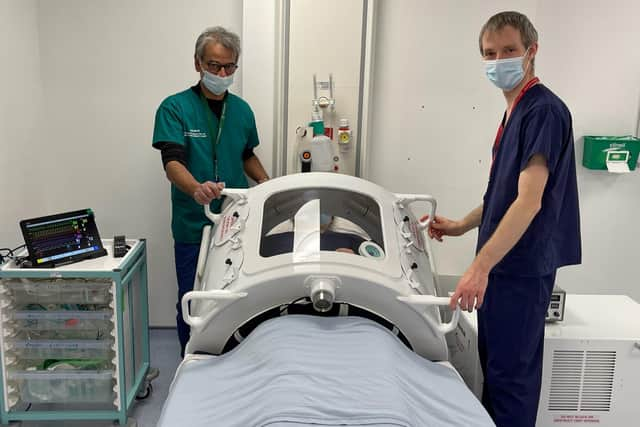
Thanks to the investment of more than £1m of volunteer time, rapid engineering development and work by Marshall Aerospace & Defence Group in partnership with WMG High Value Manufacturing Catapult, a prototype has been developed.

Advertisement
Hide AdAdvertisement
Hide AdMADG has agreed to share the knowledge and prototypes it has previously developed so that Portsmouth Aviation can take the device through regulatory approval and manufacture.
Ian Joesbury, CEO of exovent said: ‘We would like to thank Marshall Aerospace and Defence Group for their amazing work and support getting us to this advanced stage.
‘We could not have done it without them. We look forward to working with Portsmouth Aviation to start producing exovent in the UK as soon as the regulatory approval has been received and thank them for their commitment to this project.’
Exovent’s non-invasive breathing support system would offer more comfort to patients requiring mechanical ventilation, who would not need to be asleep or have an artificial airway in place.
Advertisement
Hide AdAdvertisement
Hide AdAfter the initial close monitoring of patients, the team believe the system would require less intensive nursing care and thereby the system could be used anywhere in the hospital, and even potentially at home.
Simon Escott, managing director of Portsmouth Aviation said: ‘Portsmouth Aviation is delighted to join this exciting, innovative and potentially globally lifesaving solution at a key point in its development and to take it through to manufacture. We very much look forward to becoming an integral partner and working with everybody concerned.’
Gary Moynehan, CEO of MADG, said: ‘We are now delighted to be able to hand the project over to Portsmouth Aviation and are optimistic that their own engineering and manufacturing expertise will be able to deliver a cost-effective product that will ultimately prove invaluable in the treatment of patients with a broad range of respiratory issues.’
Clinical trials are planned for later this year and it is expected that the new device will be submitted for approval by August.
Advertisement
Hide AdAdvertisement
Hide AdAssuming approval is granted by the end of the year, production and clinical trials will start next year.
For more go to portav.com/
